Where Is An Ivc Filter Placed
IVC filters help people who are recovering from accidents and surgeries by preventing potentially fatal lung clots. Doctors unremarkably place the devices in people who are at hazard for pulmonary embolism when claret thinners are ineffective or not an pick.
Doctors insert the temporary or permanent device into a patient'due south inferior vena cava, the main vein in the body that returns de-oxygenated blood from the lower body back to the heart and so to the lungs. The device resembles a metal cage. The filter's metallic wires capture and trap traveling blood clots earlier they reach the center and lungs. However, if left in besides long, the filters may cause a diverseness of serious complications.
The U.S. Nutrient and Drug Assistants canonical the device in 1979, and its use has increased continuously through the years. By 2012, doctors had inserted about 259,000 filters in patients, according to a 2016 written report in Seminars in Interventional Radiology.
Simply a 2016 analysis published by the American Higher of Cardiology found the devices were probable being over-used, retrieval rates were low and surveillance data was lacking.
"In the United States, the IVC filter implantation rates are 25 fold higher than in Europe," according to Dr. Riyaz Bashir, manager of vascular and endovascular medicine at Temple Academy Hospital. "The hospitals beyond this country collectively are spending close to a billion dollars on these devices every year without a known significant do good."
Retrievable versions of the filters should only be left in for a short time.
"The appearance of retrievable filters led to more widespread use because it was idea that they could safely exist used every bit either temporary or permanent device implants. Nonetheless, this false sense of confidence has actually led to overuse of filters. Even though retrievable filters were besides canonical for use every bit permanent devices, it turns out they may not be rubber for longterm implantation. To make matters worse, many of these filters become firmly embedded over time making them difficult to safely remove," Dr. William Kuo, Director of the Stanford IVC Filter Clinic told Drugwatch.
"Historically, most filters implanted in the U.S. have not been fairly followed for removal. By the fourth dimension the filter has been rediscovered, often years later, the devices have grown into the vein wall making them a claiming to remove safely," Dr. Kuo said.
In 2010 and again in 2014, the FDA issued safe warnings well-nigh IVC filter complications, including device migration, filter fracture, embolization, blood vessel perforation, difficulty removing the device, lower limb deep vein thrombosis and inferior vena cava occlusion.
"The FDA is concerned that retrievable IVC filters, when placed for a brusk-term risk of pulmonary embolism, are not ever removed once the risk subsides."
The agency recommends doctors consider removing retrievable filters as shortly as the run a risk of pulmonary embolism passes and usually within a 29- to 54-day window. Later that time flow, potential harm outweighs the likely benefit, according to a 2013 FDA analysis published in the Journal of Vascular Surgery.
"The FDA is concerned that retrievable IVC filters, when placed for a short-term risk of pulmonary embolism, are non always removed once the risk subsides," the agency said in a 2014 condom advice.
Attorney Holly Ennis explains the characteristics of an IVC Filter.
Filter Uses and Types
Blood clots that develop deep inside the pelvis and the lower and upper extremities are referred to as deep vein thrombosis, or DVTs.
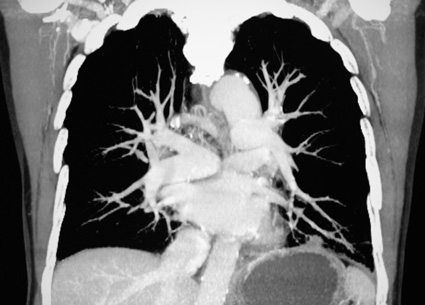
IVC Filters are designed to catch clots before they attain the heart and lungs.
DVTs can go life-threatening if they travel to the lungs and cause pregnant blockage in the lung arteries thereby interfering with oxygenation. This is known as astute pulmonary embolism, or PE. Pes cause nigh 300,000 deaths every year and they are the third nigh mutual cause of cardiovascular decease in hospital patients.
People who take claret thinner medications but notwithstanding feel DVTs and/or people who cannot tolerate blood thinners because of current bleeding or chance of bleeding are candidates for IVC filters. Doctors occasionally recommend the devices prophylactically in patients who are undergoing major surgery if at that place is a loftier gamble they may develop blood clots.
Historically, two types of IVC filters have been manufactured: permanent and retrievable. Due to the adventure of filter-related complications from prolonged implantation, permanent filters have largely been replaced by retrievable-type filters. Yet, even though retrievable filters were besides canonical for utilize equally permanent devices, it turns out they may not be safe for longterm implantation either. Therefore, both filter types may cause complications downwardly the route if not monitored closely and promptly removed..
"There are over two dozen filter types that may be encountered in patients residing in the U.Due south. The spectrum ranges from old, permanent devices inserted decades ago that have now been discontinued all the manner to the latest retrievable ones." Dr. Kuo said.
Some companies that industry devices available in the United States are ALN Implants, Argon Medical, B. Braun, BD Interventional (formerly C.R. Bard), Boston Scientific, Cook Medical, Boston Scientific, Cordis J&J, Mermaid Medical.
Some brands include:
- The Bard G2 Limited filter
- The Bard G2 filter
- The Bard Recovery filter
- The Cook Celect filter
- The Cook Gunther Tulip filter
- The Boston Scientific Greenfield filter
Placement and Removal
Doctors use a catheter to insert the device into a patient's inferior vena cava through a modest incision in either the neck or groin.
The way doctors remove retrievable filters is similar to how they implant them. Health intendance providers inject contrast or 10-ray dye in the vessel containing the device to make sure at that place are no blood clots in the filter prior to attempting removal. A catheter-similar snare is then inserted into the vein and used to engage the retrieval hook located at the end of the filter. A sheath is then inserted over the filter to collapse and remove the filter.
Lawsuit Information
Individuals are suing for blood clots, device migration and other IVC filter complications. Learn more about awaiting cases.
Possible Complications
IVC filters accept the potential to migrate abroad from their implanted location. Sometimes the device components may penetrate through the vein leading to a variety of complications. Broken pieces of filters tin can travel through the blood and guild into organs such as the middle.
"Patients should try to identify exactly which device was implanted within them, because some filters take been associated with a higher run a risk of complications including fractures, penetrations, fifty-fifty blood clots," Dr. Kuo told Drugwatch. "No device is perfect, and I have actually seen major complications occur from every filter type in existence."
Complications typically fall into three categories: procedural, delayed and retrieval.
Procedural complications may occur when doctors insert the filter. These include:
- Access site bleeding and/or bruising
- Wrong placement and/or malposition of filter
- Defective filter deployment
Retrieval complications may occur when doctors remove the filter and include:
- Bleeding
- Claret clots
- Hard retrieval causing long procedure times and backlog radiation exposure
Delayed complications may occur afterwards the filter is in the body and include:
- Filter fracture
- Filter component migration into the eye or other organs
- Filter penetration into side by side organs
- Vessel scarring with adventure of blood clots (Deep vein thrombosis)
- Vessel blockage resulting in debilitating pain and leg swelling.
Adverse Consequence Reports and FDA Actions
Betwixt 2005 and 2010, the FDA received nearly 1,000 adverse event reports involving IVC filters. Virtually involved device migration and embolization, which is a movement of the entire filter or fracture fragments to the heart or lungs. Seventy cases involved perforation of the vena cava or internal organs, and 56 involved the device breaking.
The bureau concluded the complications could have been related to retrievable filters remaining in the body after the adventure for pulmonary embolism had subsided. It recommended removing retrievable devices within ane-2 months after implantation, once the filter is no longer needed.
Madris Tomes, former FDA projection manager, highlights what makes the FDA'due south adverse event reports for IVC filters unique.
The FDA is as well requiring manufacturers to participate in studies that volition provide additional information about the prophylactic of permanent and retrievable filters. Manufacturers take been given the choice of participating in specific research, known as the PRESERVE study, or various postmarketing surveillance studies.
PRESERVE stands for PREdicting the Safe and Effectiveness of Inferior VEna Cava Filters. The independent national clinical study volition examine the rubber and effectiveness of using IVC filters to prevent pulmonary embolism. The PRESERVE study is slated for completion in May 2019.
The data gathered from the PRESERVE study and the 522 postmarketing studies will "assistance the FDA, manufacturers and health intendance professionals appraise the utilize and safety profile of these devices also as understand evolving patterns of clinical use of IVC filters, with the goal of ultimately improving IVC filter utilization and patient intendance," according to a 2016 written report in Seminars in Interventional Radiology.
However, the main limitation with PRESERVE is that the protocol only required a small-scale number of patients per filter blazon and a brusque follow-up interval overall. This PRESERVE protocol design was eerily like to the flawed trials that manufacturers initially used to obtain FDA clearance of these exact filters via the non-rigorous 510K pathway—the same pathway that has immune blessing of many other faulty devices such every bit nickel-based hip implants.
Manufacturer Recalls
6 major IVC filter recalls between 2005 and 2015 affected more than than 81,000 units. Packaging and characterization issues prompted most of the recalls, according to the manufacturers.
While there have been no major recalls since 2015, thousands of people implanted with the devices accept reported complications. And companies have not recalled some of the most problematic devices.
A 2015 NBC News investigation linked Bard Recovery and G2 filters to 39 deaths. The visitor never recalled either device. Instead, it replaced them with similar models.
Melt Medical filters also face up blame for injuries and deaths. The FDA has received hundreds of reports of Cook Celect and Gunther Tulip problems, but the visitor never issued recalls for either.
Meanwhile, thousands of people who suffered injuries take filed IVC filter lawsuits.
What Studies Say
Research studies have confirmed issues with retrievable IVC filters. A 2013 report in the Journal of the American Medical Association, or JAMA, looked at the devices' failure charge per unit. Researchers discovered doctors only removed 58 out of 679 retrievable filters. When the filters remained in patients longer than medically necessary, 18.three percent of attempts to remove the devices failed, 7.8 percent of patients had venous thrombotic events, and 25 patients suffered pulmonary embolisms.
Failure Rates
Introduced in 2003, the Recovery filter was C.R. Bard's first-generation product. A second-generation device, the Bard G2, arrived in 2005 equally a replacement for the Recovery. But earlier Bard replaced the Recovery, the FDA had received 300 reports of adverse events linked to the device.
Results from ane written report showed about 25 percent of the Recovery filters failed, causing the device to fracture or interruption apart. One patient died at home, although the report did not explicate the reason. The NBC News investigation linked the device to at least 27 deaths.
The Bard G2 had a 12 percent failure charge per unit and remained on the market a shorter amount of time than its predecessor. Bard stopped selling the Recovery when the G2 arrived on the market in 2005. The G2's successor, the G2 Express, entered the market place in 2008. One study found all of Bard's devices experienced a combined 12 percent fracture charge per unit.
'Pregnant Decline' in Use Since 2010
A 2017 study found employ of IVC filters experienced a "significant pass up" after the FDA's 2010 condom warning.
Researchers at Washington Academy School of Medicine in St. Louis looked at more 1 meg patient records roofing a ten-yr period. Between 2005 and 2010, utilize of the device rose past more than 22 percent. But information technology dropped dramatically after the FDA informational, falling more than 25 per centum by 2014.
At their peak in 2010, nearly 130,000 IVC filters per yr were placed in patients. By 2014, the number had dropped to around 96,000, according to the medical textbook, Vascular Medicine: A Companion to Braunwald's Heart Disease.
College Bloodshed Risk
A written report that appeared in JAMA Network Open up in 2018 institute an association between IVC filters and increased take chances of decease. The study looked at 126,000 patients' medical records and found the take chances of dying within thirty days of getting a filter shot up 18 percent for sure patients.
Those at risk had 2 specific weather: they had venous thromboembolic illness, or VTE, disease and they could non have blood thinners. VTE includes deep vein thrombosis, pulmonary embolism or both.
The researchers called for randomized clinical trials to better test the filters' rubber and effectiveness.
Other Treatment Options
IVC filters should primarily exist used in patients with astute blood clots who cannot have blood thinners. If filters are used for any other reason, this should only exist done afterward conscientious consideration and appropriate documentation, experts say. Otherwise, filters should non be widely used, and most patients with claret clots should simply be treated with blood thinners, close monitoring and lifestyle modifications.

Pradaxa, an example of a blood thinner alternative to IVC filter
Eliquis, an example of a blood thinner alternative to IVC filter
Historically, the almost widely used blood thinner was warfarin, but newer anticoagulants similar Xarelto, Pradaxa and Eliquis are increasingly being prescribed. All blood thinners are associated with an increased hazard for bleeding, then patients should be closely monitored, especially those patients who are prescribed life-long claret thinners.
Where Is An Ivc Filter Placed,
Source: https://www.drugwatch.com/ivc-filters/
Posted by: maserneash1938.blogspot.com


0 Response to "Where Is An Ivc Filter Placed"
Post a Comment